Stop for migration!
Summary Text
Collaborative research groups have found that glycerol phosphate modification of glycans is exhibited in various cancer tissues, and this modification disrupts glycan-mediated cell adhesion, thereby promoting the migration of cancer cells. Their findings can contribute to the development of cancer therapies targeting this modification.
Collaborative research groups have found that glycerol phosphate modification of glycans is exhibited in various cancer tissues, and this modification disrupts glycan-mediated cell adhesion, thereby promoting the migration of cancer cells. Their findings can contribute to the development of cancer therapies targeting this modification.
Full Text
Dystroglycans on epithelial cell surfaces interact with the extracellular matrix via long sugar chains (called matriglycans), and they are responsible for cell adhesion. Abnormalities in the formation of matriglycans can impair cell adhesion and cause muscular dystrophy.
The collaborative groups, including researchers at Nagoya City University and National Institutes of Natural Sciences, have previously discovered the presence of a novel post-translational modification, in which glycerol phosphate (GroP) caps the core part of matriglycan, thereby blocking its elongation.
In this recent study, the researchers have shown that GroP modification is enhanced in cancer tissues. Moreover, this modification is promoted as cancer progresses. They established a human colon cancer cell line with increased GroP expression by overexpressing a bacterial enzyme that synthesizes CDP-Gro, a key precursor of GroP modification. They have also demonstrated that these cells have an increased cell migration capacity. Furthermore, they have found a high correlation in colorectal cancer tissue between GroP modification and the expression of PCYT2, a CDP-Gro synthase in humans. These findings indicate that enhanced GroP modification in cancer cells can terminate matriglycan elongation, thereby disrupting cell adhesion, which in turn promotes the migration of cancer cells.
Their findings provide insights into cancer therapy: if enhanced GroP modification can promote malignant transformation of cancer cells, then cancer metastasis can be inhibited by antagonizing this modification. Proteins involved in GroP modifications, such as PCYT2, are considered as potential targets for drug discovery. In addition, antibodies targeting GroP modification could be developed into antibody-based therapeutics against cancer.
Dystroglycans on epithelial cell surfaces interact with the extracellular matrix via long sugar chains (called matriglycans), and they are responsible for cell adhesion. Abnormalities in the formation of matriglycans can impair cell adhesion and cause muscular dystrophy.
The collaborative groups, including researchers at Nagoya City University and National Institutes of Natural Sciences, have previously discovered the presence of a novel post-translational modification, in which glycerol phosphate (GroP) caps the core part of matriglycan, thereby blocking its elongation.
In this recent study, the researchers have shown that GroP modification is enhanced in cancer tissues. Moreover, this modification is promoted as cancer progresses. They established a human colon cancer cell line with increased GroP expression by overexpressing a bacterial enzyme that synthesizes CDP-Gro, a key precursor of GroP modification. They have also demonstrated that these cells have an increased cell migration capacity. Furthermore, they have found a high correlation in colorectal cancer tissue between GroP modification and the expression of PCYT2, a CDP-Gro synthase in humans. These findings indicate that enhanced GroP modification in cancer cells can terminate matriglycan elongation, thereby disrupting cell adhesion, which in turn promotes the migration of cancer cells.
Their findings provide insights into cancer therapy: if enhanced GroP modification can promote malignant transformation of cancer cells, then cancer metastasis can be inhibited by antagonizing this modification. Proteins involved in GroP modifications, such as PCYT2, are considered as potential targets for drug discovery. In addition, antibodies targeting GroP modification could be developed into antibody-based therapeutics against cancer.
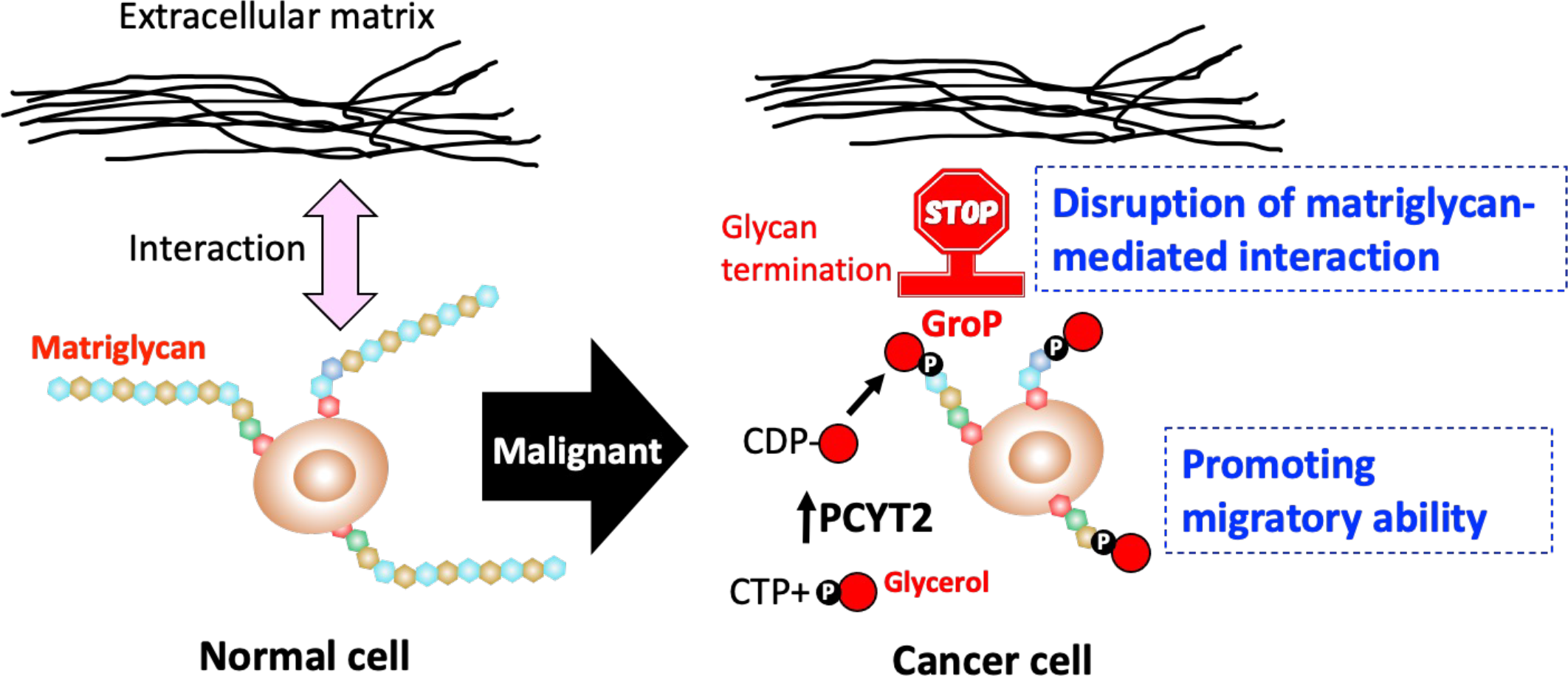
IMAGE: Increased expression of PCYT2, which synthesizes CDP-Gro in cancer, enhances GroP modification, and impairs matriglycan formation. This phenomenon allows cancer cells to leave the constraints of the extracellular matrix and gain migratory capacity.
Author
Hirokazu Yagi, Koichi Kato
Hirokazu Yagi, Koichi Kato


